In second grade science, students investigate ways that matter can change and whether these changes are reversible. In this post, I’ll show you a fun changing matter experiment you can easily do at school by making ice cream in a bag! It’s a delicious demonstration of how temperature can change matter from a liquid to a solid that students can eat once they finish!
Changing Matter Experiment for Second Grade
Did you know that ice cream is a solid, a liquid, and a gas all at once? It’s true. Ice cream is a combination of solid ice crystals, liquid milk fat, and air bubbles. Making ice cream is a fun and easy way for students to investigate how matter is changed by heating and cooling. As they make their ice cream, students also gain experience in carrying out investigations, making observations, and collecting and analyzing data.
To get started you will need a few inexpensive items that are readily available at any grocery store. I even found a half gallon size jug of half-and-half.
Materials per student:
- 1 cup half-and-half per student
- 2 Tbs. sugar
- 1/2 tsp. vanilla extract
- 3 cups ice
- 1/3 cup rock salt or kosher salt
- sandwich size zipper bag
- 1 gallon size zipper bag per 2 students
- plastic spoons
- student lab sheet
Multiply the recipe by the number of students you have. I recommend making a large batch of this recipe in a pitcher ahead of time.
1. Mix the half-and-half, sugar, and vanilla extract together in a pitcher.
2. Pour about a cup of the mixture in each student’s sandwich bag.
3. Fill a gallon size Ziploc bag about half full of ice. Add 1/3 cup of salt.
4. Place 2 sealed, sandwich bags with the mixture in each large bag. Seal the bag firmly.
5. Students take turns shaking the bag vigorously for about 7-10 minutes, pausing to record changes they observe on their lab sheet.
Source: Changing Matter Experiment
Analyzing the Changing Matter
Have students describe the state of their mixture at the start. Next, they record changes they observed after cooling and shaking the mixture for 1 minute, 5 minutes, and 10 minutes. After the investigation, have students compare their data with other students to determine an average time it takes to freeze the mixture. Have students discuss whether they think the amount of shaking is a factor.
As students are completing their lab sheets, encourage them to describe the properties of their ice cream including color, shape (the mixture takes the shape of its container while liquid), and texture.
I recommend making an extra bag of ice cream to demonstrate reversing the change. This can be done by placing the ice cream bag in a sunny window or leaving it out on a counter to melt.
The Science Behind Ice Cream
Adding salt to ice lowers its melting point. The ice absorbs heat from its surroundings (the bag of ice cream mixture) which allows the ice cream mixture to freeze. Since the mixture isn’t water it needs to be a little below 32 degrees to freeze. Adding salt allows the temperature around the mixture to get colder.
Find more properties of matter science activities in this unit: Properties of Matter unit for 2nd grade .

Get Free Science Activities
Try these engaging science lessons at home or in the classroom! This FREE science mini-unit includes both printable + digital activities on Google Slides that make it easy to learn science no matter where you are!
I hope you will try this changing matter experiment and give your students hands-on experience in demonstrating a reversible change. Be sure to pin this post for later so you have it when you plan!

Visit these posts for more hands-on, high engagement science activities:
Marvelous Ways to Teach Matter

Happy teaching!

 Changing matter experiment
Changing matter experiment 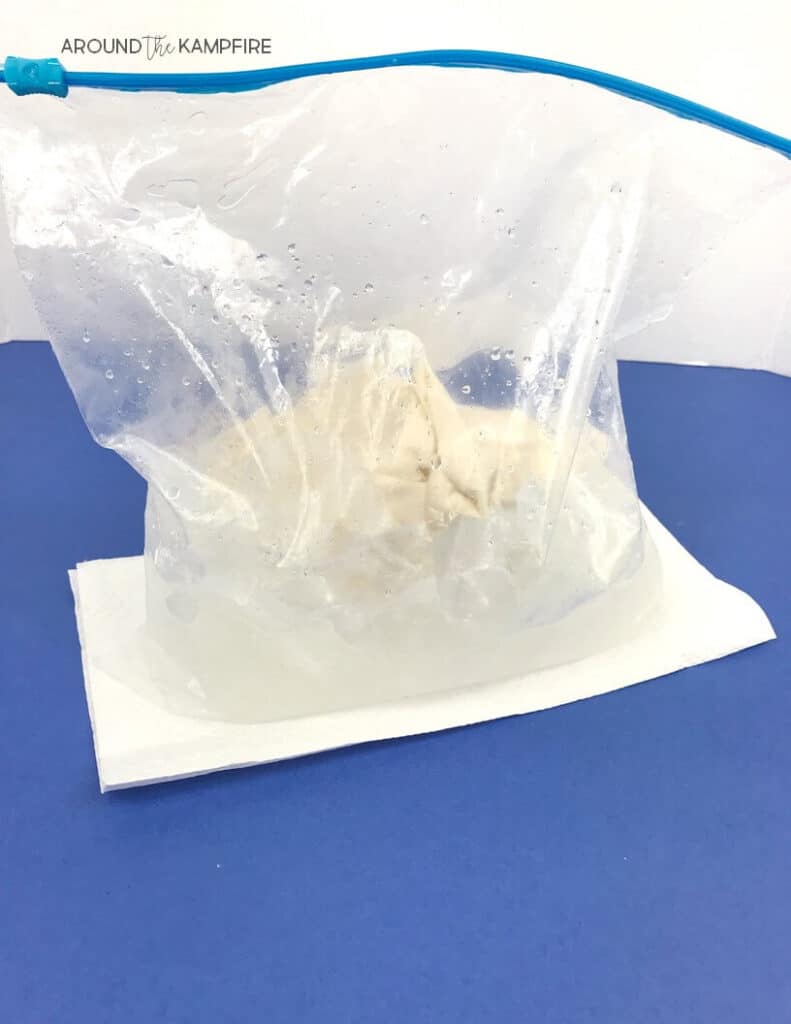










Hi! Student teacher here. Is there any way I can purchase just this ice cream activity? Thank you in advance! Would love to do this with my second grade class.
Hi! I have a student who is dairy free. Do you know if this would work with dairy-free half and half? Thanks!
Hi Sarah,
I haven’t tried making ice cream with dairy free half and half. If you do try it, I’d love to know if it works!
Is there a printable with this for collecting the data for the ice cream experiment?
Hi. Where can I get a copy of the lab sheet?
Thanks for sharing this interactive and hands-on experiment!
You’re welcome Esther! I hope your students enjoy it!
Is there any way to buy the ice cream recording sheets and not the entire matter unit? We already taught matter but it was when we were virtual. I want to do this experiment after field day next week! (Trying to make this year as normal as possible!)